Point Of Care Devices For Covid

Point of care antigen test devices on july 14 centers for medicare and medicaid cms announced an initiative to distribute rapid point of care poc antigen covid 19 testing devices to nursing homes across the country.
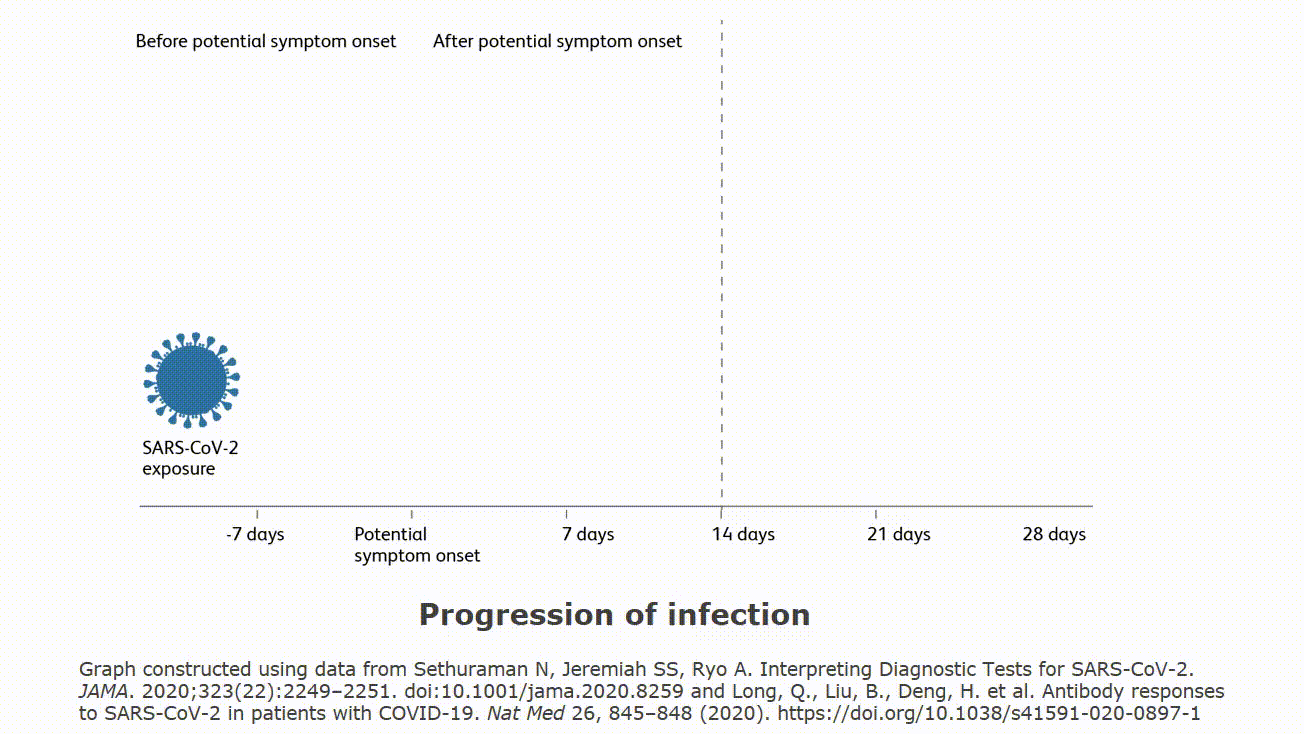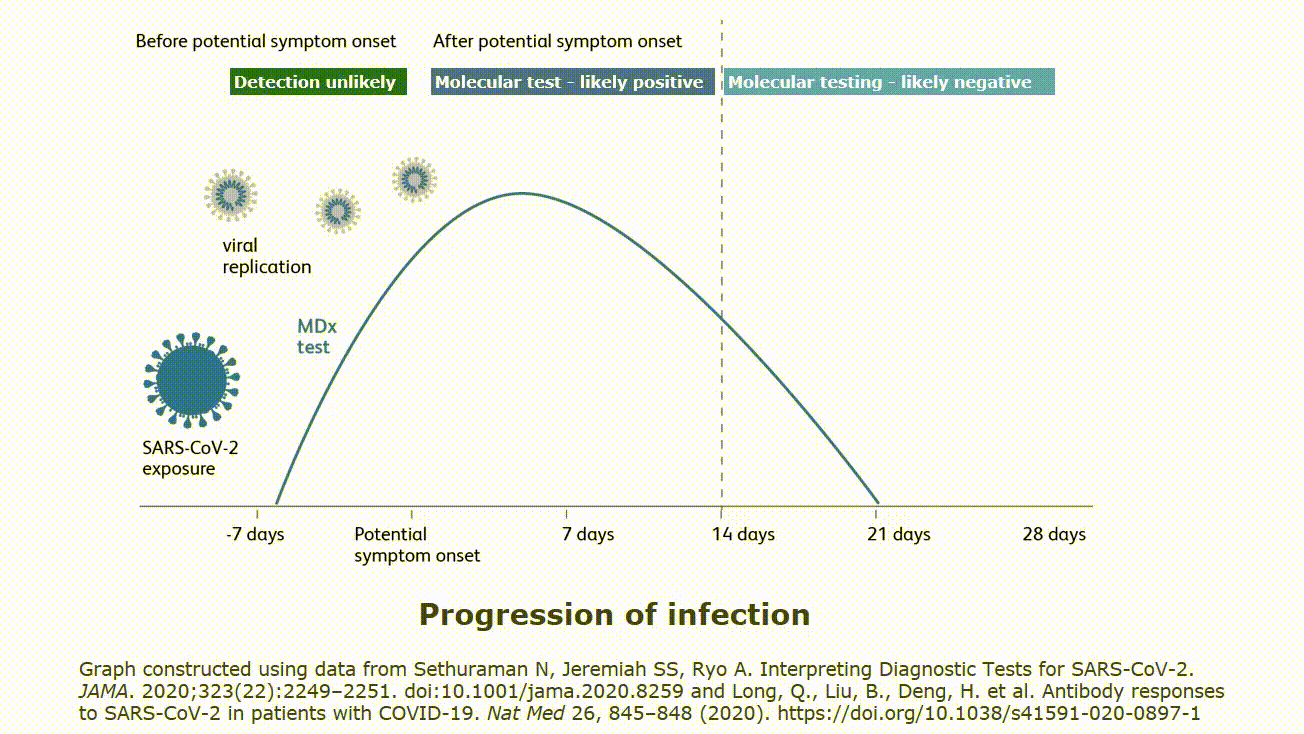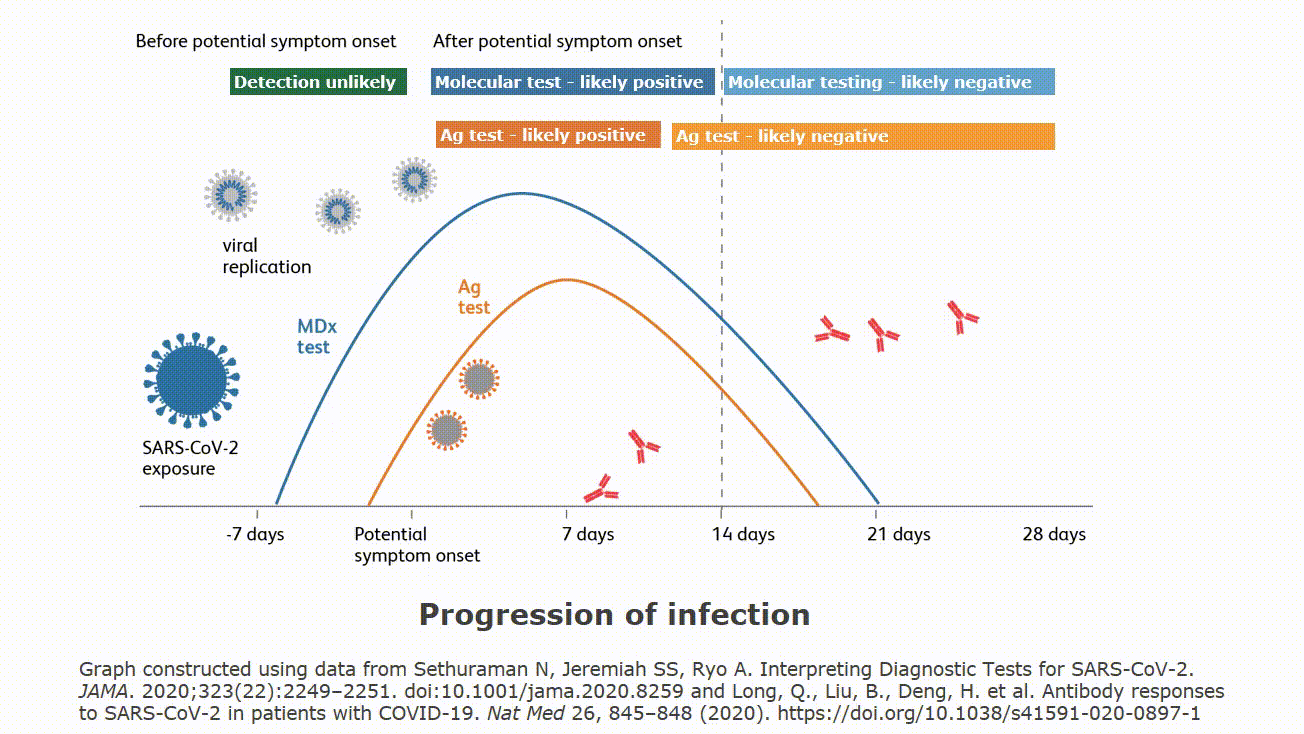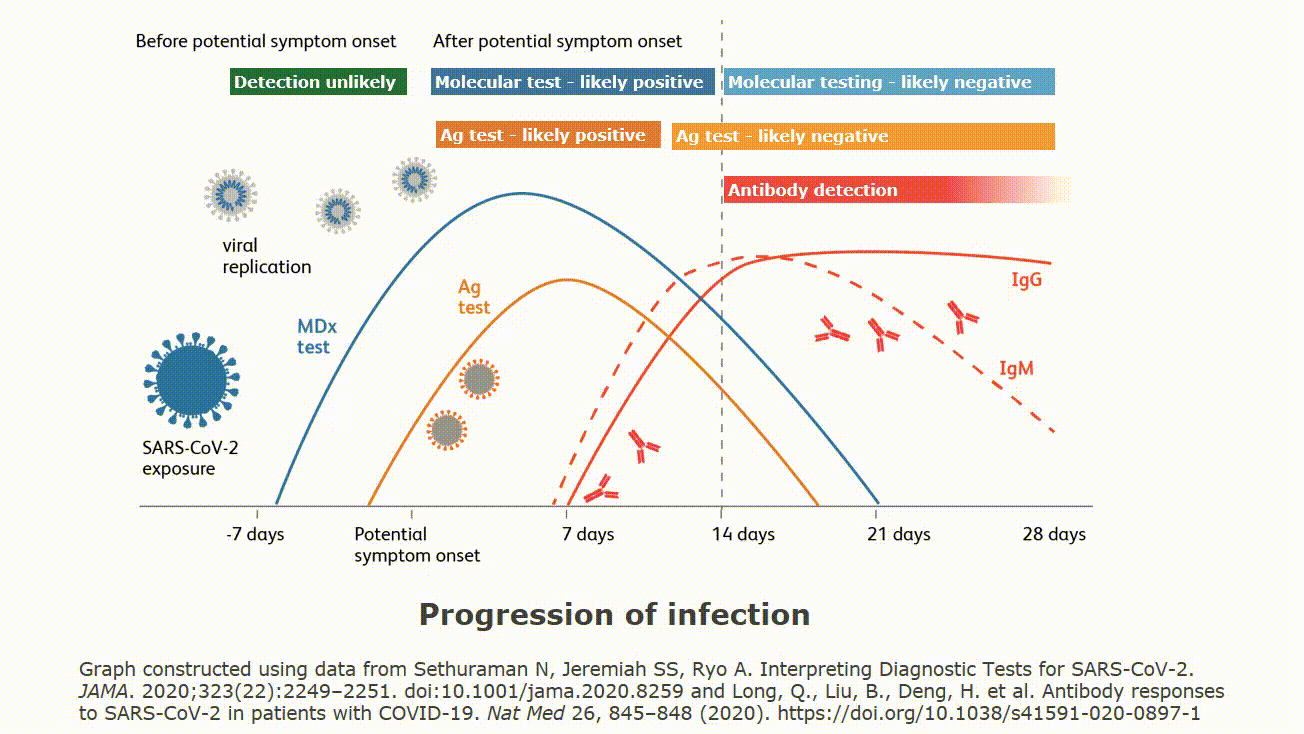
Point of care devices for covid. The assure covid 19 igg igm rapid. They cannot test saliva or blood. Food and drug administration issued an emergency use authorization eua for the first serology antibody point of care poc test for covid 19. All three of these tests require nasal or throat specimens on swabs.
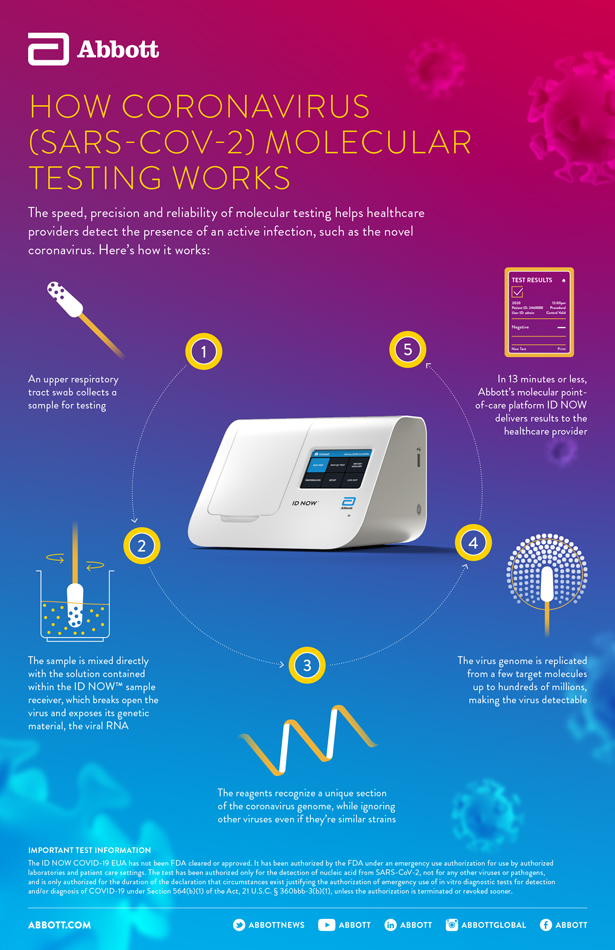
Point of care tests. Awaiting response from manufacturer. Pathogendx united states. Molecular testing technologies help detect the presence of a virus by identifying a small section of the virus genome then amplifying that portion until there s enough for detection.
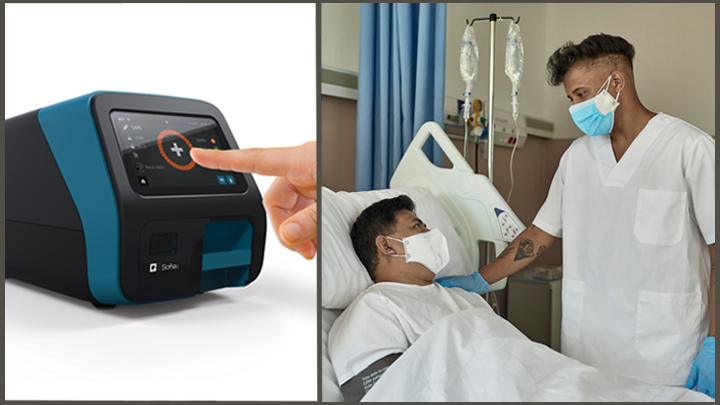
Press release public statement. Covid 19 diagnostic device applications received by health canada device name manufacturer device type footnote 1 laboratory or point of care test footnote 1 status. Quidel sofia 2 sars antigen fia. The fda issued the first emergency use authorization for a point of care covid 19 diagnostic for the cepheid xpert xpress sars cov 2 test.
All three of these tests require nasal or throat specimens on swabs. Point of care tests. As of april 29 fda has granted emergency use authorization for three commercial products with covid 19 tests that can be run in patient care settings i e. They cannot test saliva or blood.
Point of care test. Molecular point of care testing for covid 19 offers healthcare workers rapid results in more settings where people show up for care. Nursing facilities will receive one of two testing devices. Point of care reverse pcr tests as of april 29 fda has granted emergency use authorization for three commercial products with covid 19 tests that can be run in patient care settings i e.
Point of care reverse pcr tests. Thousands of nursing homes across the country will be given point of care covid 19 tests by the administration starting next week officials announced tuesday. South korea antigen technology. Food and drug administration emergency use authorization eua.