Point Of Care Devices For Covid 19

Point of care test.
Point of care devices for covid 19. At the end of 2019 the novel coronavirus disease covid 19 a fast spreading respiratory disease caused by severe acute respiratory syndrome coronavirus 2 sars cov 2 was reported in wuhan china and has. Molecular point of care testing for covid 19 offers healthcare workers rapid results in more settings where people show up for care. 23 2020 prnewswire today the u s. Food and drug administration issued an emergency use authorization eua for the first serology antibody point of care poc test for covid 19.
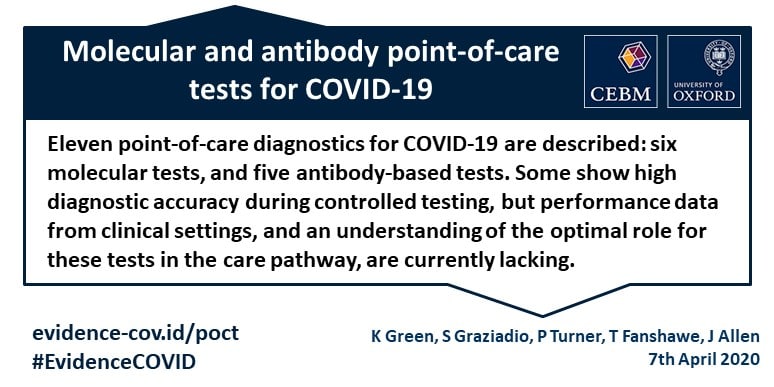
In addition to high and moderate complexity. Eleven diagnostic tests that are potentially suitable for testing for covid 19 at the point of care are described. Point of care test. The assure covid 19 igg igm rapid test device can now be used in doctors offices hospitals urgent care centers and emergency departments to provide immediate results.
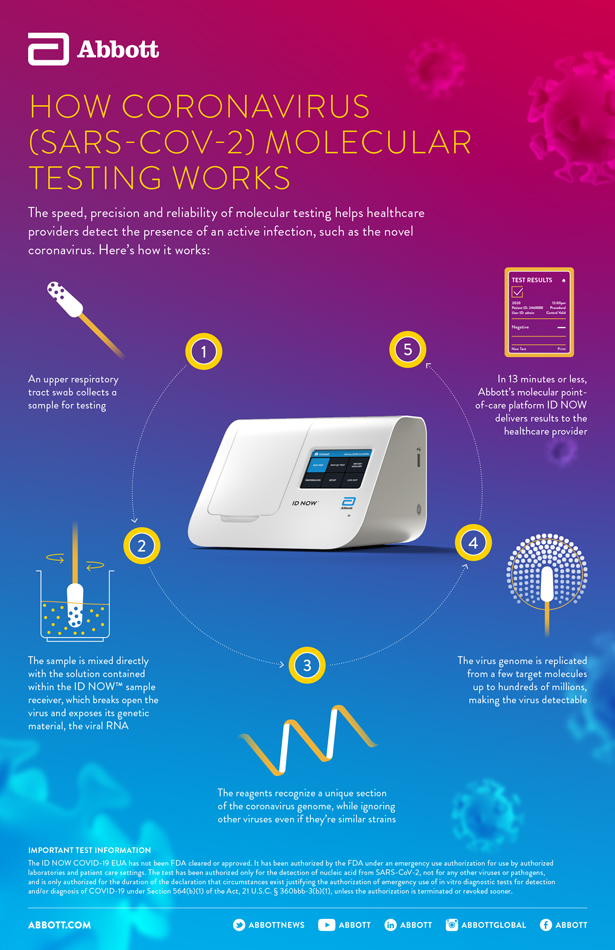
Molecular testing technologies help detect the presence of a virus by identifying a small section of the virus genome then amplifying that portion until there s enough for detection. Canada nucleic acid technology. Point of care rna based diagnostic device for covid 19 diagnostics basel. Some devices show high diagnostic accuracy during controlled testing but performance data from clinical settings and a clear understanding of the optimal population and role for.
Food and drug administration on friday issued emergency use authorization for the first point of care test for diagnosing covid 19. Covid 19 igm igg antibody test. Covid 19 igg igm rapid test device. Covid 19 nucleic acid detection kit fluorescence pcr esenso biotech inc.
Press release point of care testing poct devices market analysis 2020 covid 19 impact and global analysis by key companies comprehensive analysis by global industry share size growth. Silver spring md sept. Food and drug administration issued an emergency use authorization eua for the first serology antibody point of care poc test for covid 19.